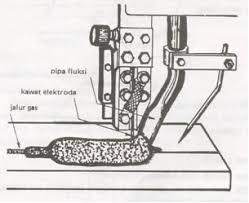
Here is some information about welding metallurgy. I hope you enjoy this one...
Aku dapat materi ini sewaktu berkunjung (sebenarnya sih lebih k jalan-jalan daripada berkunjung ;) ). Jadi jika mau ngopi mohon sekiranya dipergunakan dengan baik dan benar ya. Aku g ingin kamu mendapat karma gara-gara daku yang hina ini..hehehe
Dan akhirnya monggo dinikmati dan sugeng berkreasi untuk kemajuan las di Indonesia..
Ooiya ada yang lupa,silahkan di perbanyak jika diperlukan..Absolutelly Free..
Vivat LAS..!!!
CSM